Bohr Diagram Of Sulfur
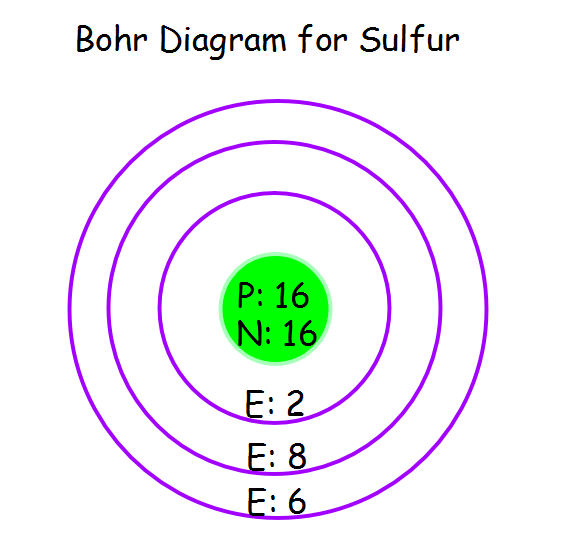
The sulfur atom has 16 protons, 16 neutrons and 16 electrons in three different energy levels, or orbits. Physics suggests that electrons do not physically exist as "points," but teachers use the Bohr atom model with fixed electrons as a way to simplify atomic structure. Creating the model requires the ability to cut with scissors and use glue.

Sulfur atom tewswheel
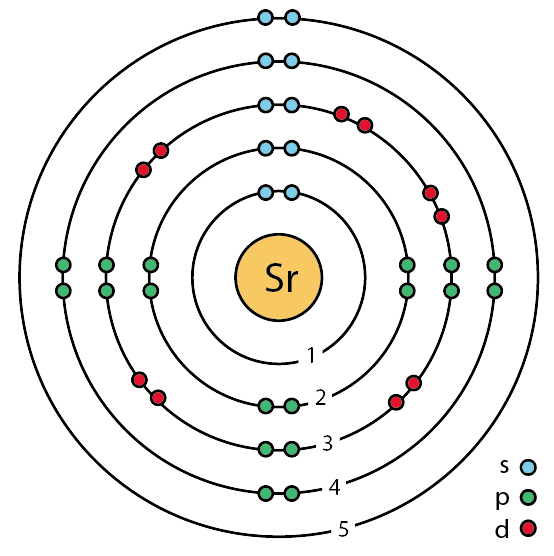
Bohr model of calcium: (CC BY-SA 2.0 uk;Greg Robson): Answer b. Bohr model of sulfur: (CC BY-SA 2.0 uk; Greg Robson). Valence electrons are located in the highest energy level of an atom. When drawing a Bohr diagram, the valence electrons would be present in the outermost electronic level/shell (furthest away from the nucleus).
:max_bytes(150000):strip_icc()/sulfuratom-58b602563df78cdcd83d5a9d.jpg)
Atom Diagrams Electron Configurations of the Elements
The atomic number of sulfur represents the total number of electrons of sulfur. Since the atomic number of sulfur is 16, the total electrons of sulfur are 16. Calculate the maximum number of electrons each subshell can hold using the formula: 4ℓ + 2. Finally, use aufbau chart and start writing electron configuration.
Bohr Diagram Of Sulfur
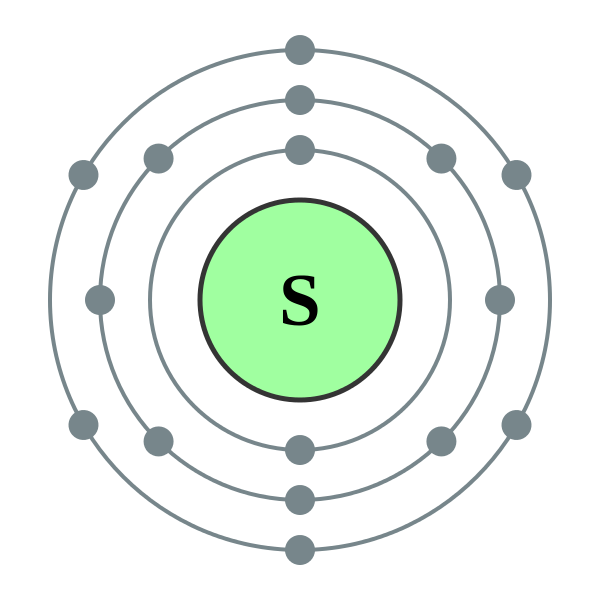
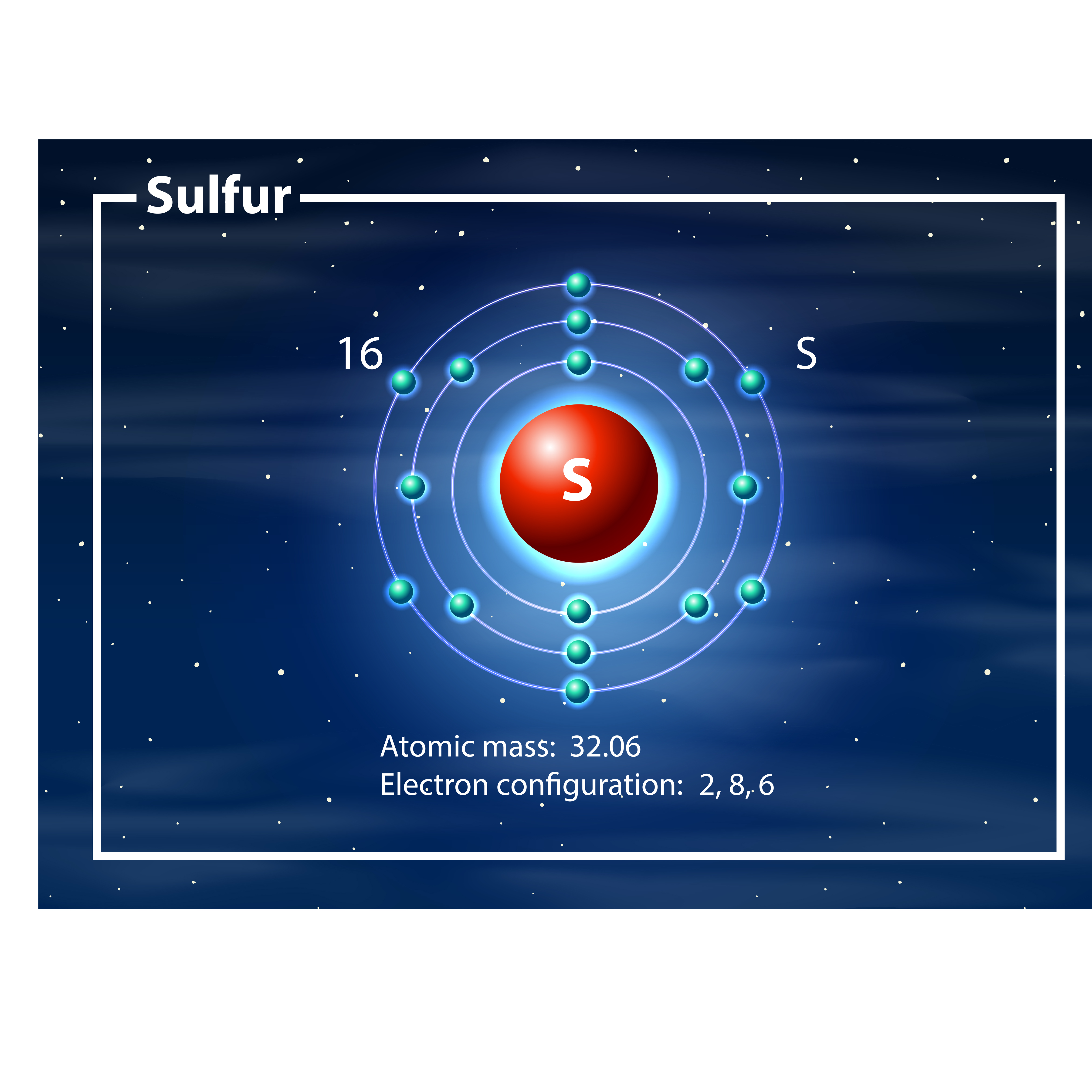
The Bohr model of Sulfur (S) is drawn with three electron shells, the first shell contains 2 electrons, the second shell contains 8 electrons and the third shell contains 6 electrons. Sulfur is neutral and its atomic number is 16, hence, the number of protons and electrons available for its Bohr diagram is also 16.
Sulfur Table of Elements by Shrenil Sharma
The Bohr Rutherford diagram of sulfur illustrates that it has 16 electrons distributed in different energy levels or shells around the nucleus. The first energy level contains two electrons, and the second energy level contains eight electrons. The outermost or valence shell contains six electrons.

Bohr Diagram Of Sulfur
In this video we'll look at the atomic structure and Bohr model for the Sulfur atom (S). We'll use a Bohr diagram to visually represent where the electrons a.

Sulfur Bohr Model — Diagram, Steps to Draw Techiescientist
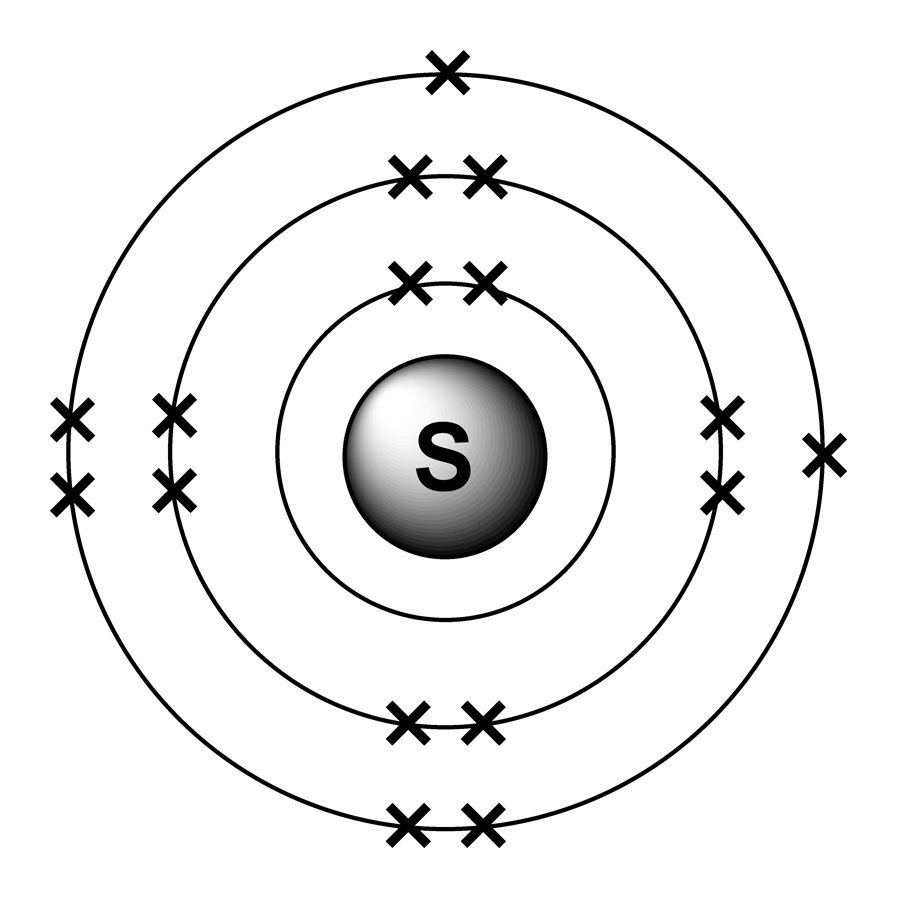
Sulfur / Sulphur has 2 electrons in its first shell, 8 in its second, 6 in its third.Check me out: http://www.chemistnate.com

Diagram representation of the element sulfur Vector Image
On the far left of Figure 3.6.1 3.6. 1 are the highest energy electromagnetic waves. These are called gamma rays and can be quite dangerous, in large numbers, to living systems. The next lower energy form of electromagnetic waves are called x-rays. Most of you are familiar with the penetration abilities of these waves.
Bohr Diagram The Element Sulfur
You have already seen the bohr model of sulfur element in the above table. From the Bohr model, it can be found that the number of orbits or shells in sulfur is 3. Hence, as sulfur has 3 orbits, it lies in period 3 of the Periodic table.. Orbital Diagram of All Elements (Diagrams given Inside) Subscribe to our newsletter. Subscription Form.
Chemist atom of sulfur diagram 528624 Vector Art at Vecteezy
Bohr's expression for the quantized energies is: En = − k n2, n = 1, 2, 3,.. E n = − k n 2, n = 1, 2, 3,.. In this expression, k is a constant comprising fundamental constants such as the electron mass and charge and Planck's constant. Inserting the expression for the orbit energies into the equation for Δ E gives.

Unit 1 Chemistry in Action Lesson 4 Bohr Diagrams ppt video
Here's how you can draw the Bohr model of sulfur step by step. #1 Write protons, neutrons, and electrons of sulfur atom. #2 Draw nucleus of sulfur atom. #3 Draw 1 st electron shell. #4 Draw 2 nd electron shell. #5 Draw 3 rd electron shell. Let's break down each step in detail.
Sulfur Bohr Model Diagram My XXX Hot Girl
Bohr model of Elements. 1. Hydrogen (H) 1. 2. Helium (He) 2. 3. Lithium (Li)
Electron Configuration Noble Gas Atom Bohr Model Chemistry Sulfur
6.2 The Bohr Model; 6.3 Development of Quantum Theory; 6.4. The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as. phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their.

Sulfur Atom Science Notes and Projects
Drawing the Bohr Model of Sulfur. Sulfur is the 16 th element of the Periodic table. It belongs to Period 3 and group 16. The information that we can infer from the above-mentioned Sulfur box is as follows: • The atomic number of Sulfur is 16. • The electronic configuration of Sulfur is [Ne] 3s 2 3p 4.
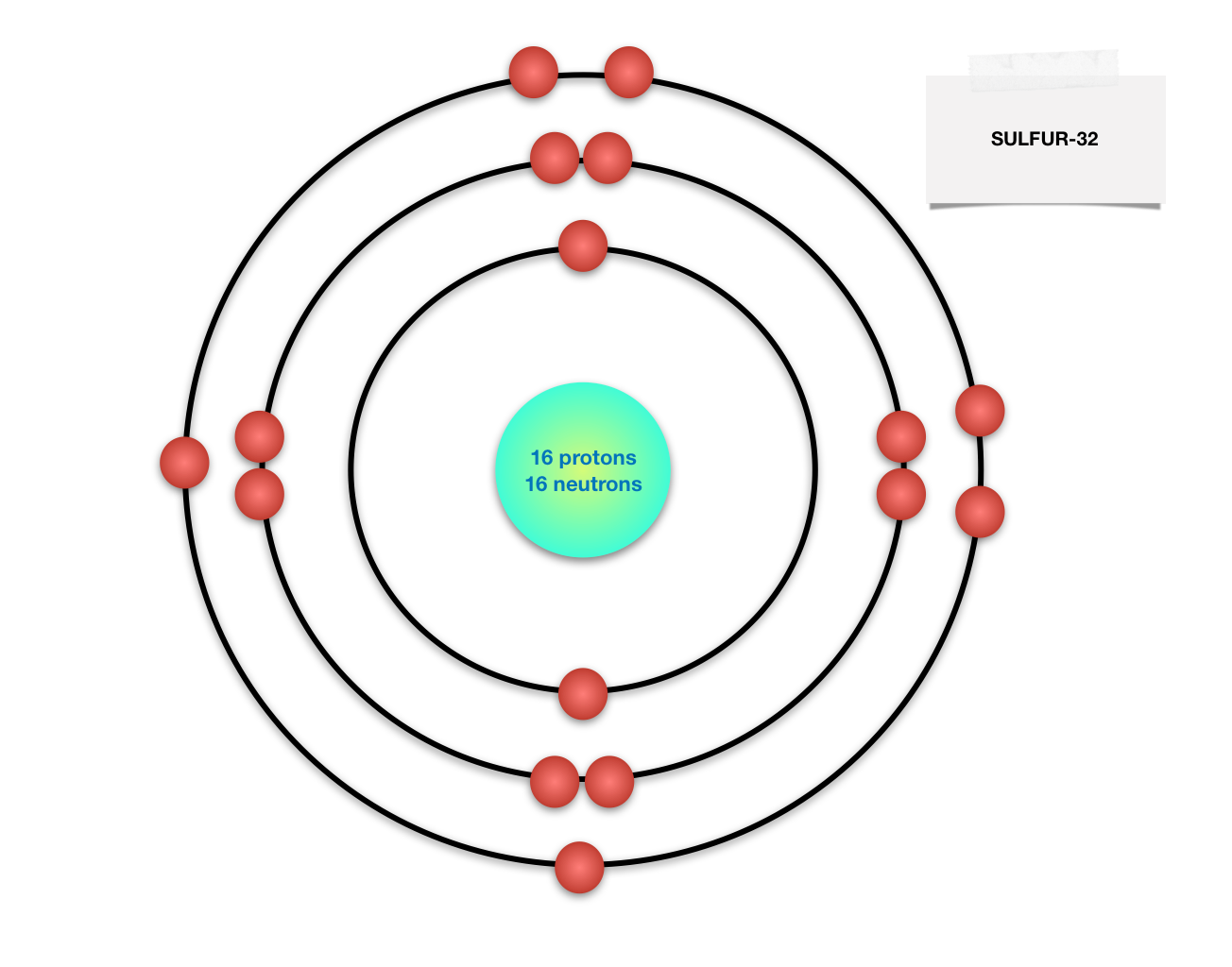
Draw BohrRutherford diagram for the sulfur32 atom. Quizlet
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.

Sulfur Atom Diagram General Wiring Diagram
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons